TIRF
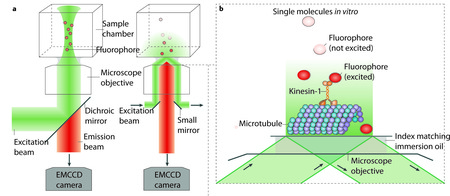
Single-molecule fluorescence microscopy to study motor proteins
(a) Epi-illumination (left) and total-internal-reflection fluorescence (TIRF) illumination (right) are shown. The excitation beam (green) and backwards emitted emission light (orange) are separated by a dichroic mirror in the case of epi-illumination, and by a dichroic mirror or small mirrors at the edge of the objective in the case of TIRF illumination. An electron multiplying charged coupled device (EMCCD) camera is used for low noise imaging at low light levels (for example, for detecting single fluorophores).
(b) TIRF concentrates the illumination light to a thin layer near the surface. Single fluophores (orange) that are attached to kinesins moving along surface-attached MTs can be imaged selectively, while fluorophores in the background, further away from the surface, remain unexcited (dark grey spheres).
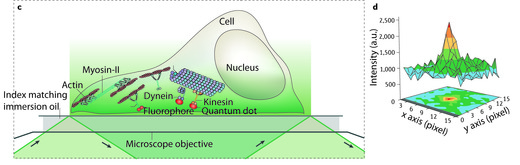
(c) When applied to cells, TIRF only illuminates a thin layer where cells are attached to the surface of the chamber. Again only the fluophores near the surface of the chamber are excited and detected. Large numbers of motors are active in cells, as schematically indicated, but only those near the surface will be visible.
(d) 2D intensity profile of an image of a single deac-aminoADP attached to a cover slip. The maximum of the intensity clearly peaks over the background noise. The centre of the maximum, and thus the location of the fluorophore, can be determined with an uncertainty limited by the standard error of the mean of the distribution. Figure in part d reproduced with permission from Sakamoto et al. Nature 455, 128-131.