Optical Tweezers
Experimental Setup
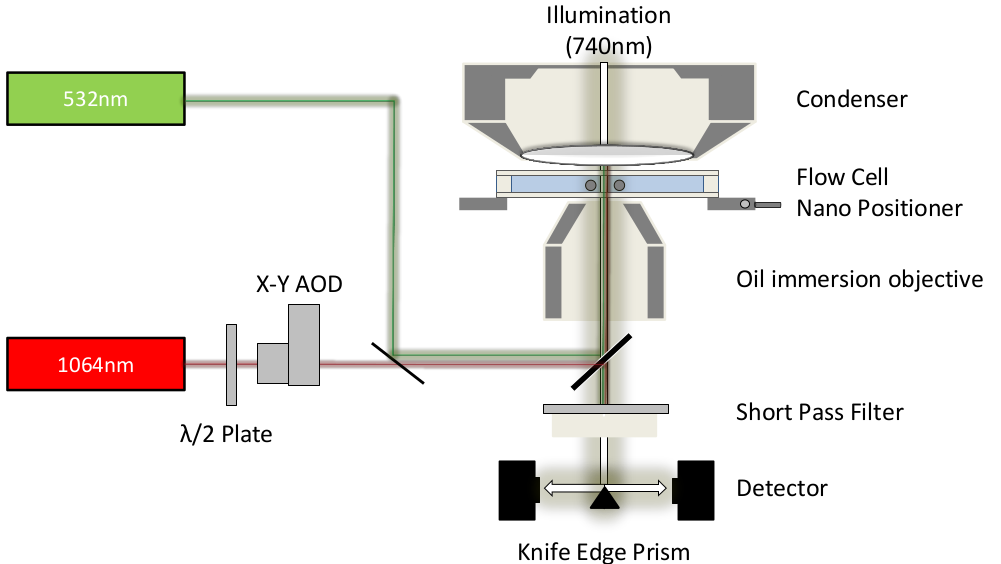
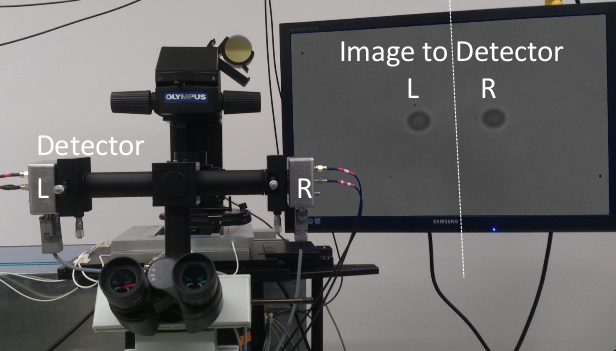
Two four-quadrant photodetectors are being used to measure the position of both beads holding the actin filament by spliting the microscope image with a knife edge prism mirror.
In our lab we are equipped with two optical tweezers that have a very fast detection (µs) due to a custom-build force detector. Furthermore, both traps can run independently without any need for a timesharing of the AOD. Our traps has also a built-in TIRF (532nm).
In order to detect gold-nanoparticles, we installed a laser-backscattering setup (647nm)
Principle
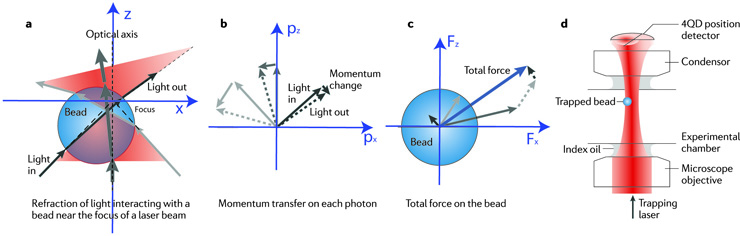
(a) Ray optics sketch of light rays incident from different directions that are refracted through an optically trapped bead that is positioned slightly off the optical axis, and below the focus of a strongly focused laser (red cones). The width of the arrows indicates the light intensity, which is highest on the optical axis (as represented by the thickest arrow).
(b) The deflection of each photon results in a momentum change and a transfer of momentum onto the bead. Since wavelength does not change, the magnitude of the momenta is unchanged (as indicated by the equal length of arrows). Arrows between solid (original photon momentum) and dashed (deflected photon momentum) arrows show the momentum transfers for individual photons out of the three rays of light, drawn in colours corresponding to part a.
(c) The total momentum transfer per time on all deflected photons in the three rays equals the force exerted on the bead (indicated by the blue arrow), which is the sum of the forces produced by the three rays. The gradient of intensity in the laser cone implies more photons per time impinging near the optical axis, that is, the dark grey arrow is longer.
(d) Principle of high-bandwidth position and force detection by back-focal-plane interferometry, using a quadrant photodiode (4QD position detector). The quadrant diode (4QD position detector) is placed right behind the microscope condenser so that it can collect the deflected laser light and detect angular shifts in the transmitted trapping light.
Publications containing optical tweezers data:
- Mechanics and Activation of Unconventional Myosins. Batters C, Veigel C: Traffic. Vol. 17(8) (2016) Aug
- Myosin-10 produces its power-stroke in two phases and moves processively along a single actin filament under low-load. Takagi Y, Farrow RE, Billington N, Nagy A, Batters C, Yang Y, Sellers JR and Molloy JE: Proc Natl Acad Sci USA 2014 May 6;111(18):E1833-42 (2014)
- Using optical tweezers to study the fine details of Myosin ATPase mechanochemical cycle. Batters C, Veigel C. Methods Mol Biol 778:97-109 (2011)
- Moving into the cell: single-molecule studies of molecular motors in complex environments. Veigel C, Schmidt CF. Nature Reviews Mol Cell Biol 12:163-176 (2011).
- Drug effect unveils inter-head cooperativity and strain-dependent ADP release in fast skeletal actomyosin. Albet-Torres N, Bloemink MJ, Barman T, Candau R, Frölander K, Geeves MA, Golker K, Herrmann C, Lionne C, Piperio C, Schmitz S, Veigel C, Månsson A. J Biol Chem. 284:22926-37 (2009).
- Load-dependent kinetics of myosin-V can explain its high processivity. Veigel C, Schmitz S, Wang F, Sellers JR. Nature Cell Biol. 7:861-9 (2005).
- A model of stereocilia adaptation based on single molecule mechanical studies of myosin I. Batters C, Wallace MI, Coluccio LM, Molloy JE. Philos Trans R Soc Lond B Biol Sci. 359: 1895-905 (2004).
- A monomeric myosin VI with a large working stroke. Lister I, Schmitz S, Walker M, Trinick J, Buss F, Veigel C, Kendrick-Jones J. EMBO J. 23: 1729-38 (2004).
- Neck length and processivity of myosin V. Sakamoto T, Wang F, Schmitz S, Xu Y, Xu Q, Molloy JE, Veigel C, Sellers JR. J Biol Chem. 278: 29201-7 (2003).

